Your Custom Text Here
The Challenge
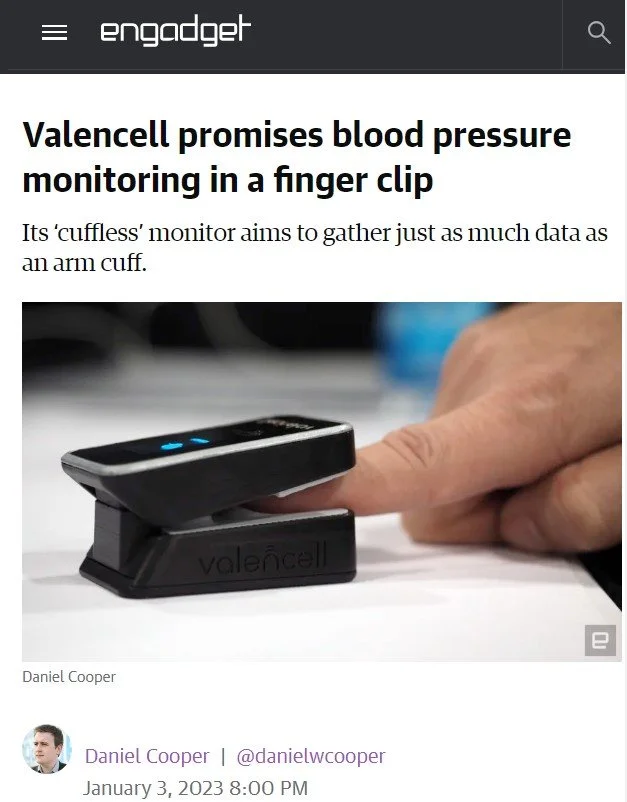
After successfully making an initial announcement of the first cuffless and calibration-free blood pressure device that will be able to take measurements from the fingertip, Valencell wanted to stay in the public eye, and Tandem was tasked with keeping the momentum created at CES going.
The Solution
As leaders in the field of BP monitoring, the aim was to position and secure Valencell spokespeople as commentators on feature interviews and as authors of several thought leadership opportunities.
Our aim was to ensure that the media were kept focused on the changing BP monitoring industry and why it needs a change. As part of that positioning exercise, we had to tell the narrative of how and why Valencell is moving from a sensor company to a device manufacturer.
The Result
Throughout the CES period, Valencell achieved 21 media placements in outlets such as T3, PharmaPhorum, and Healthcare IT Today, along with several interviews in shows such as The Peggy Smedley Show. Over the following six months, more than four byline articles have been placed, and Valencells spokespeople have undertaken several speaker slots at events.
Find other case studies here:
Morphée: Relax, Meditate, And Sleep: The Unconnected Way
Withings U-Scan: As Easy As 1,2, Pee!
Withings Horizon: Elegance and Style In A Hybrid SmartWatch
Allstate Protection Plans: Breakability Samsung Galaxy S23
Bringing Assistive Technology And ChatGPT To Blind And Low-Vision Communities
SXSW Take Over: Withings Activité Pop